The incorporation of biomarkers into clinical trials has the potential to improve trial efficiency, reduce required sample sizes, and increase statistical power to detect treatment effects. By identifying and measuring reliable biomarkers early in the trial, researchers can refine patient selection, stratify risk, and potentially replace or supplement traditional endpoints with more responsive or biologically relevant measures. Despite these advantages, questions remain regarding the appropriate study design, analysis plan, and methodological strategies to ensure that incorporating biomarkers truly enhances the power of a trial rather than inflating Type I error or introducing bias.
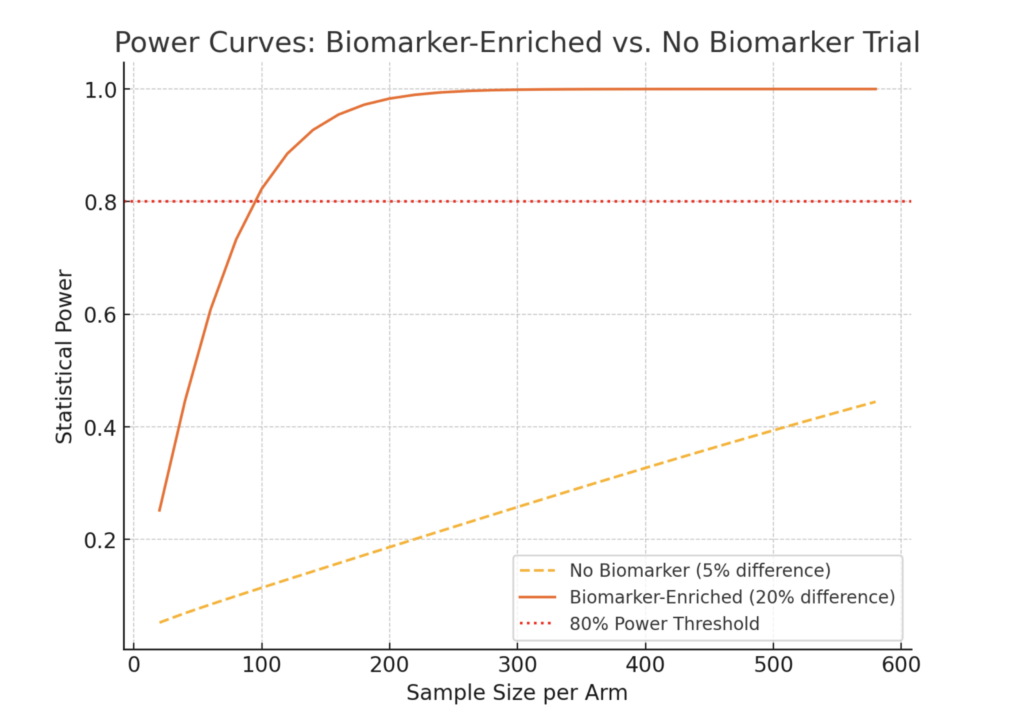
Above, I show sample power curves for clinical trials informed by biomarkers where they are prognostic and predictive in nature.
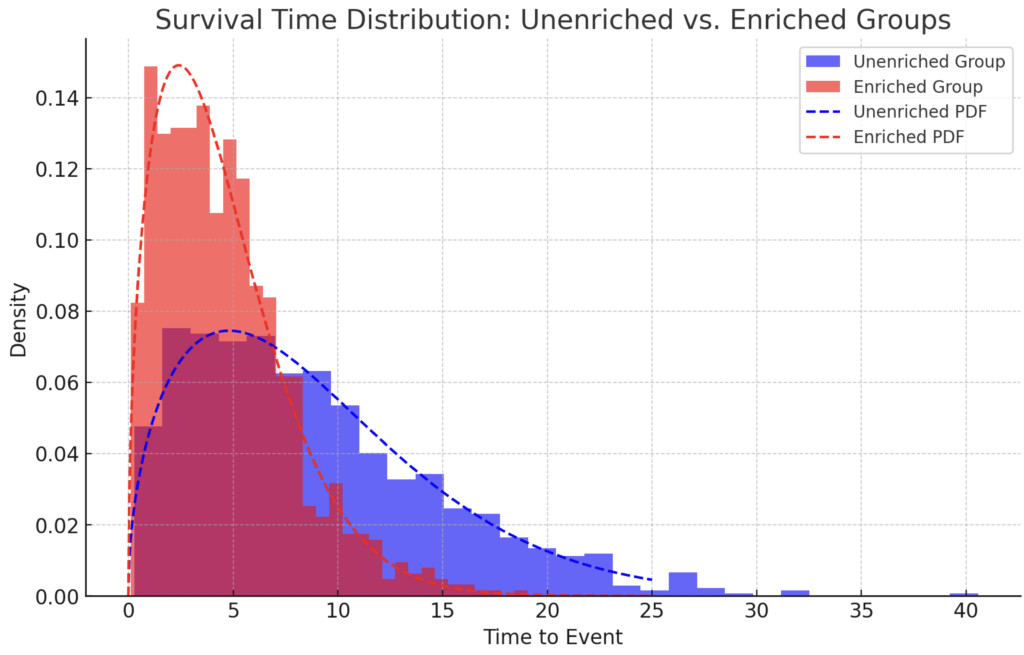
Biomarkers can also be used to increase the event rate of clinical trials. Genetic risk scores can be used as a very powerful biomarker for clinical trials.
Leave a Reply